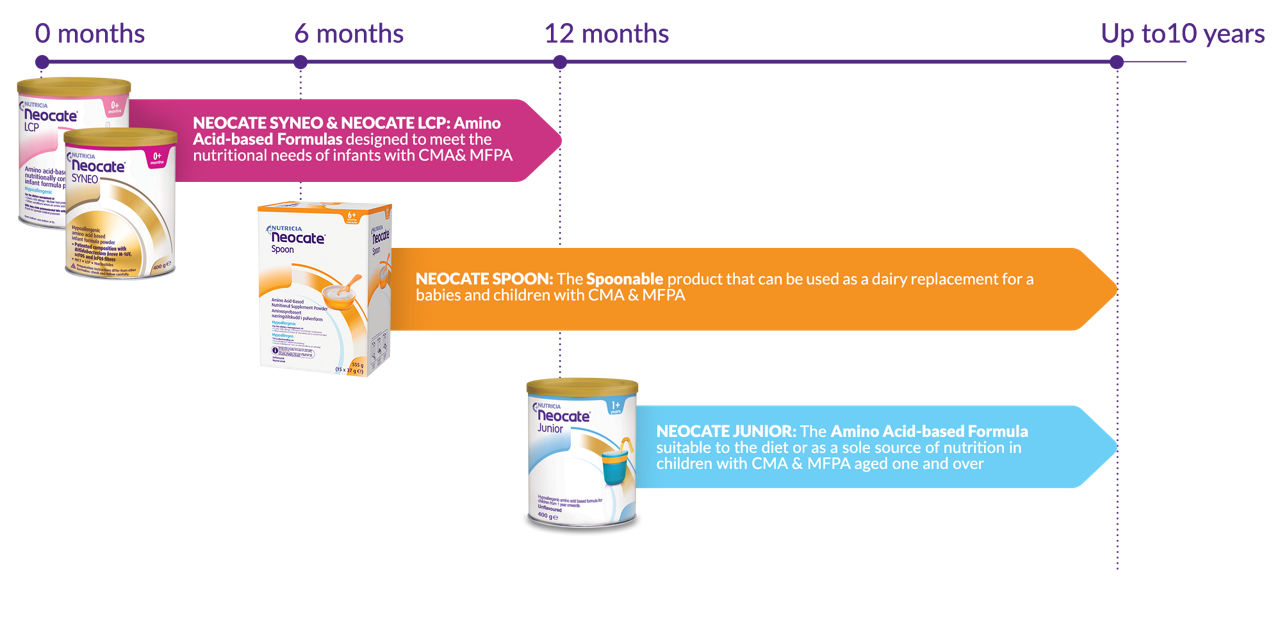
The Neocate range
Neocate is the only Amino Acid-based Formula range for infants and children from birth to 10 years*. Neocate is supported by over 90 publications and over 35 years of clinical experience.

About Neocate
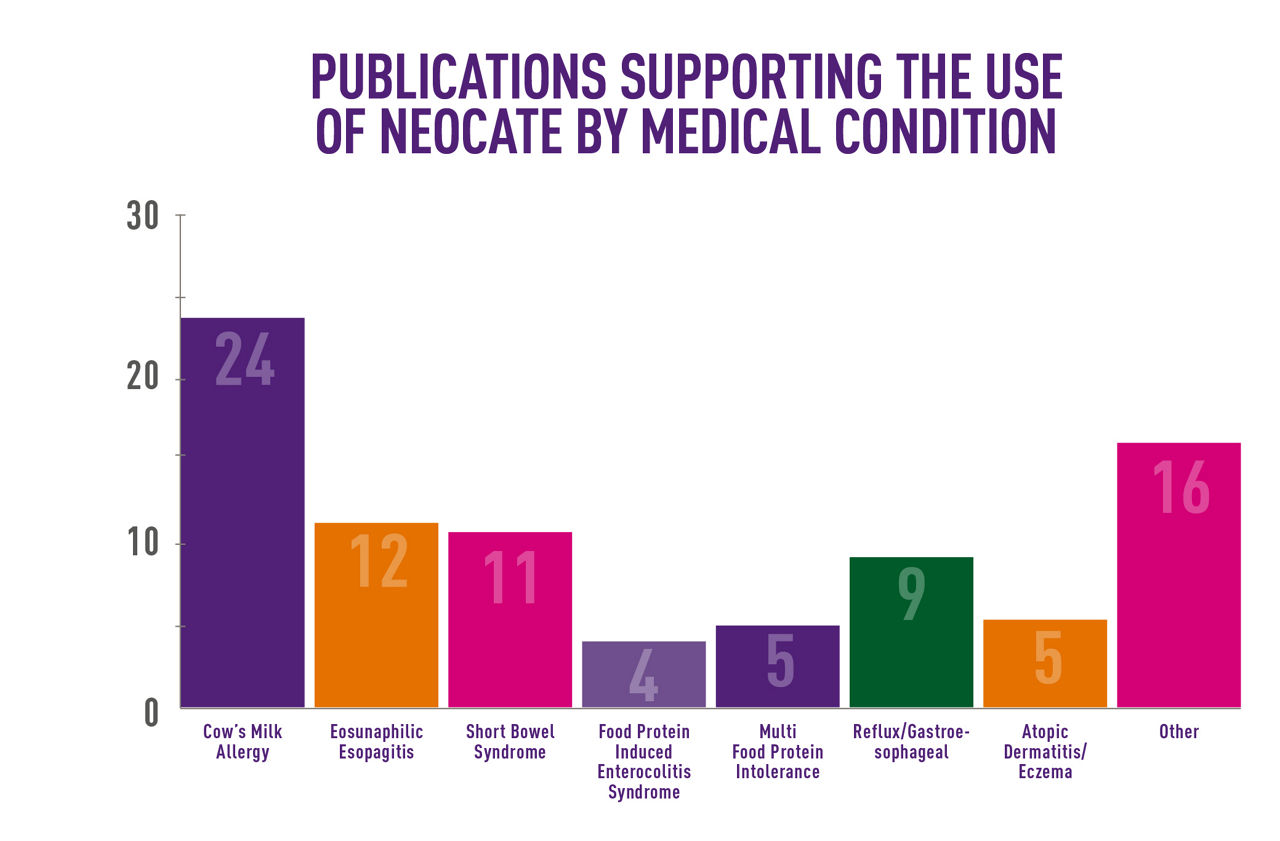
- Neocate has a strong evidence based heritage with 35 years clinical experience and 90 publications.
- Neocate is made up of a non-allergenic base of 100% free amino acids, and is manufactured in a milk free environment.
Neocate range
Neocate recognises the importance of evidence based practice. Research studies using Neocate have been presented at leading scientific meetings and published in expert peer-reviewed journals. Neocate has over 35 years clinical experience and over 90 publications which support the use of Neocate in a range of medical conditions.
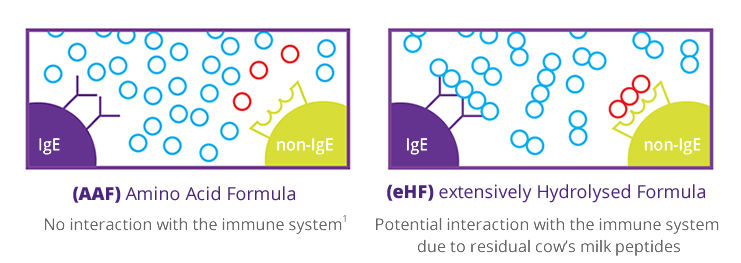
Amino acids do not interact with the immune system and therefore do not lead to an allergic response1.
The hypoallergenicity of Neocate has been established as per the American Academy of Pediatrics (AAP) 1990 recommendations. The AAP recommendations are also endorsed by the European Society of Paediatric Gastroenterology and Nutrition (ESPGHAN) and the European Society of Paediatric Allergy and Clinical Immunology (ESPACI)2.
Using the above criteria, Neocate was tested in infants and children with hypersensitivity to cow's milk. Sampson et al. showed that Neocate is ''hypoallergenic'' and is well tolerated by children who are allergic to cow's milk3.
Neocate is an effective formula for the dietary management of CMA and MFPA in thousands of infants and toddlers in the UK and around the world.
- Host & Halken, Allergy 2004; 59(78): 45-52.
- Høst A et al. Dietary products used in infants for treatment and prevention of food allergy. Joint Statement of the European Society for Paediatric Allergology and Clinical Immunology (ESPACI) Committee on Hypoallergenic Formulas and the European Society for Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) Committee on Nutrition. Arch Dis Child. 1999 Jul;81(1):80-4. Review
- Sampson, H.A et al. Safety of an amino acid derived infant formula in children allergic to cow milk. Pediatrics. 1992 90; (3) 463-5.
*MIMS, November 2020.
Help us provide information most relevant to you
Please ensure your role and areas of interest are up to date.